A) Δ S° > 0, Δ H° > 0
B) Δ S° > 0, Δ H° < 0
C) Δ S° < 0, Δ H° < 0
D) Δ S° < 0, Δ H° > 0
E) Δ G° > 0
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In 1774 Joseph Priestley prepared the element oxygen by heating mercury(II) oxide: HgO(s) → Hg(l) + ½O2(g) For this reaction, ΔH° = 90.84 kJ and ΔS° = 108 J/K. Which of the following statements is true?
A) The reaction is only spontaneous at low temperatures.
B) The reaction is spontaneous at all temperatures.
C) ΔG° becomes less favorable as the temperature is raised.
D) The reaction is spontaneous only at high temperatures.
E) The reaction is spontaneous under standard conditions at 25°C.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship or statement best describes ΔS° for the following reaction? Pb(s) + Cl2(g) → PbCl2(s)
A) Δ S° ≈ 0
B) Δ S° < 0
C) Δ S° > 0
D) Δ S° = Δ H°/T
E) More information is needed to make a reasonable prediction.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following pairs will the first system have a higher entropy than the second? Assume P and T are the same for each pair, unless stated otherwise.
A) 1 mole He( g) ; 1 mole Kr( g)
B) 1 mole O 2( g) ; 2 mole O( g)
C) 1 mole CH 4( g) ; 1 mole C 2H 6( g)
D) 1 mole Xe( g) at 1 atmosphere; 1 mole Xe( g) at 0.5 atmosphere
E) 20 one-dollar bills distributed randomly among 20 people; 20 one-dollar bills distributed randomly among 10 people
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The higher the pressure of a gas sample, the greater is its entropy.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship or statement best describes ΔS° for the following reaction? BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
A) Δ S° ≈ 0
B) Δ S° < 0
C) Δ S° > 0
D) Δ S° = H°/ T
E) More information is needed to make a reasonable prediction.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitric oxide reacts with chlorine to form NOCl. The data refer to 298 K.2NO(g) + Cl2(g) → 2NOCl(g)  What is the value of ΔG° for this reaction at 550 K?
What is the value of ΔG° for this reaction at 550 K?
A) -143.76 kJ
B) -78.78 kJ
C) -22.24 kJ
D) -10.56 kJ
E) 66600 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction CuI(s) ⇄ Cu+(aq) + I−(aq) If the concentrations of the Cu+ and I− ions in equilibrium at 298 K are both equal to 1.03 × 10−6 M, what is the value of ΔG° for the reaction?
A) −68 kJ
B) 68 kJ
C) −30 kJ
D) 30 kJ
E) 34 kJ
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship best describes ΔS° for the following reaction? 8H2(g) + S8(s) → 8H2S(g)
A) Δ S° = Δ H°
B) Δ S° = Δ H°/ T
C) Δ S° ≈ 0
D) Δ S° < 0
E) Δ S° > 0
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The free energy of a perfect crystal at absolute zero, is zero.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
For a reaction at equilibrium, ΔSuniv = 0.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate ΔS° for the reaction 2Cl2(g) + SO2(g) → SOCl2(g) + Cl2O(g)
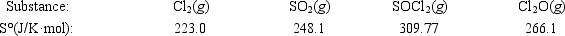
A) −118.2 J/K
B) −104.8 J/K
C) 104.8 J/K
D) 118.2 J/K
E) 1270.0 J/K
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a chemical reaction to be non-spontaneous at any temperature, which of the following conditions must be met?
A) Δ S° > 0, Δ H° > 0
B) Δ S° > 0, Δ H° < 0
C) Δ S° < 0, Δ H° < 0
D) Δ S° < 0, Δ H° > 0
E) All reactions are spontaneous at some temperature.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship or statement best describes ΔS° for the following reaction? O3(g) + NO(g) → O2(g) + NO2(g)
A) Δ S° ≈ 0
B) Δ S° < 0
C) Δ S° > 0
D) Δ S° = ΔH°/T
E) More information is needed to make a reasonable prediction.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction is proceeding toward equilibrium. At a certain stage, the concentrations of reactants and products are such that ΔG = ΔG°. What conclusion can reasonably be drawn about the reaction at this time?
A) K > Q
B) K < Q
C) K = Q
D) K = 1
E) Q = 1
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
Under a given set of conditions, all microstates of a system are equally probable.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship or statement best describes ΔS° for the following reaction? HgS(s) + O2(g) → Hg(l) + SO2(g)
A) Δ S° ≈ 0
B) Δ S° < 0
C) Δ S° > 0
D) Δ S° = Δ H°/ T
E) More information is needed to make a reasonable prediction.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship or statement best describes ΔS° for the following reaction? 2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g)
A) Δ S° ≈ 0
B) Δ S° < 0
C) Δ S° > 0
D) Δ S° = Δ H°/ T
E) More information is needed to make a reasonable prediction.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship or statement best describes ΔS° for the following reaction? CaO(s) + CO2(g) → CaCO3(s)
A) Δ S° ≈ 0
B) Δ S° < 0
C) Δ S° > 0
D) Δ S° = Δ H°/ T
E) More information is needed to make a reasonable prediction.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
For any reaction, if ΔG° > 0, then K < 1.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 85
Related Exams