A) − 2.31 V
B) +4.33 V
C) − 1.32 V
D) +2.00 V
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
Secondary cells are rechargeable.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
If an electrochemical half-reaction includes the production or consumption of a gas, the standard state is a pressure of 1 atm.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most prevalent type of primary battery in use today is _____.
A) the rechargeable batter y
B) the lead-acid storage battery
C) the alkaline batter y
D) the nickel-metal-hydride battery
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the cell potential (E 0 ) for a galvanic cell formed from the following two half- reactions ? 
A) −1 .43 V
B) +1.43 V
C) −0 .93 V
D) +0.93 V
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rusting of the sheet metal of a car is an example of:
A) uniform corrosion.
B) galvanic corrosion.
C) electrolysis.
D) galvanized steel.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species undergoes reduction in the formation of iron (III) oxide?
A) FeO
B) Fe2O3
C) Fe
D) O2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Galvanic corrosion occurs only when two different metals contact each other in the presence of an appropriate electrolyte.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the context of cell notations in galvanic cells, a phase boundary is denoted by _____.
A) |
B) ||
C) -
D) =
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
A positive voltage in the standard reduction potential means that the half-reaction proceeds as a reduction when connected to the standard hydrogen electrode (SHE)because the SHE is serving as the anode .
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species undergoes oxidation in the formation of iron (III) oxide?
A) FeO
B) Fe2O3
C) Fe
D) O2
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
A galvanic cell is an electrochemical cell in which a spontaneous chemical reaction can be used to generate an electrical current.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Single use, non-rechargeable batteries are referred to as _____.
A) primary cells
B) secondary cells
C) tertiary cells
D) electrolytic cells
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify a true statement about a redox reaction.
A) It involves a transfer of protons from one species to another.
B) It creates electrons.
C) It involves a transfer of electrons between two species.
D) It is always nonspontaneous.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following galvanic cell: 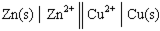 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:
A) spontaneous half of the reaction.
B) oxidation half-reaction.
C) anode of the cell.
D) reduction half-reaction.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most common fuel cells are based on the reaction of _____.
A) nickel and oxygen to produce nickel oxide
B) hydrogen and oxygen to produce water
C) lead and sulfuric acid to produce lead sulfate
D) zinc and air to produce zinc oxide
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cells is most likely to be an example of a galvanic cell?
A) Zn(s) | Zn 2+ || Cu 2+ | Cu(s)
B) Cu(s) | Cu 2+ || Fe 2+ | Fe(s)
C) Au(s) | Au + || Ag + | Ag(s)
D) Sn(s) | Sn 2+ || Fe 2+ | Fe(s)
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose that you have an iron rod that requires copper coating. You have calculated that you need to deposit 4.00 g of copper to achieve an adequate coating. If your electrolysis cell (using Cu 2+) runs at 1.5 A, how long must you operate the cell to obtain the desired coating?
A) 1 hour and 12 minutes
B) 2 hours and 15 minutes
C) 3 hours and 22 minutes
D) 4 hours and 35 minutes
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the Nernst equation?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electrolysis cell that deposits copper (from Cu 2+ ions) operates for 30 minutes at an electric current of 3.20 A. What mass of copper is deposited?
A) 1.9 g
B) 2.3 g
C) 3.5 g
D) 4.2 g
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 45
Related Exams