A) 24
B) 156
C) 294
D) 43
E) 39
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li (s) + N2 (g) → 2Li3N (s) In a particular experiment,2.50-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
A) 2.51
B) 2.09
C) 12.5
D) 4.18
E) 6.2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Combustion of a 0.9827-g sample of a compound containing only carbon,hydrogen,and oxygen produced 1.900 g of CO2 and 1.070 g of H2O.What is the empirical formula of the compound?
A) C2H5O
B) C5H7O3
C) C4H11O2
D) C4H10O
E) C2H5O2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound that is composed of only carbon and hydrogen contains 85.7% C and 14.3% H by mass.What is the empirical formula of the compound?
A) CH2
B) C2H4
C) CH4
D) C4H8
E) C86H14
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lead (II) carbonate decomposes to give lead (II) oxide and carbon dioxide: PbCO3 (s) → PbO (s) + CO2 (g) ________ grams of lead (II) oxide will be produced by the decomposition of 8.75 g of lead (II) carbonate?
A) 10.5
B) 2.50
C) 0.033
D) 7.31
E) 8.75
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 30.5 gram sample of glucose (C6H12O6) contains ________ mol of glucose.
A) 0.424
B) 0.169
C) 5.90
D) 2.36
E) 0.136
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula weight of PbCO3 is ________ amu.
A) 279.2
B) 112.0
C) 118.0
D) 267.2
E) 235.2
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
1 mole of which of the following will have the largest mass?
A) rubidium
B) magnesium
C) gold
D) neon
E) 1 mole of any element will contain the same mass.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
A certain alcohol contains only three elements,carbon,hydrogen,and oxygen.Combustion of a 60.00 gram sample of the alcohol produced 114.6 grams of CO2 and 70.44 grams of H2O.What is the empirical formula of the alcohol?
Correct Answer

verified
Correct Answer
verified
Short Answer
The combustion of propane (C3H8)in the presence of excess oxygen yields CO2 and H2O: C3H8 (g)+ 5O2 (g)→ 3CO2 (g)+ 4H2O (g) How many grams of CO2 is produced when 12.5 g of C3H8 burns in open air?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
GeF3H is formed from GeH4 and GeF4 in the combination reaction: GeH4 + 3GeF4 → 4GeF3H If the reaction yield is 89.1%,how many moles of GeH4 are needed to produce 3.50 mol of GeF3H?
A) 15.7
B) 3.93
C) 0.982
D) 0.875
E) 2.95
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced,the coefficient of HNO3 is ________. HNO3 (aq) + CaCO3 (s) → Ca(NO3) 2 (aq) + CO2 (g) + H2O (l)
A) 1
B) 2
C) 3
D) 5
E) 4
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced,the coefficients are ________. 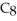
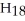 + O2 → CO2 + H2O
+ O2 → CO2 + H2O
A) 4, 50, 16, 18
B) 1, 5, 2, 2
C) 2, 2, 7, 1
D) 1, 13, 8, 9
E) 2, 25, 16, 18
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A nitrogen oxide is 63.65% by mass nitrogen.The molecular formula could be ________.
A) NO
B) NO2
C) N2O
D) N2O4
E) either NO2 or N2O4
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percentage by mass of carbon in CO2.
A) 27.29
B) 75.10
C) 73.05
D) 72.71
E) 37.53
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percentage by mass of hydrogen in PtCl2(NH3) 2.
A) 1.558
B) 1.008
C) 0.672
D) 0.034
E) 2.016
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced,the coefficients are ________. Al(NO3) 3 + Na2S → Al2S3 + NaNO3
A) 2, 3, 1, 6
B) 2, 1, 3, 2
C) 1, 1, 1, 1
D) 4, 6, 3, 2
E) 2, 3, 2, 3
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: 4 NH3 (g) + 7 O2 (g) → 4 NO2 (g) + 6 H2O (g) The combustion of 14.4 g of ammonia consumes ________ g of oxygen.
A) 13.5
B) 28.8
C) 54.1
D) 47.3
E) 94.6
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li (s) + N2 (g) → 2Li3N (s) How many moles of lithium are needed to produce 0.45 mol of Li3N when the reaction is carried out in the presence of excess nitrogen?
A) 0.23
B) 1.4
C) 0.15
D) 0.30
E) 2.7
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the empirical formula of a compound that contains 49.4% K,20.3% S,and 30.3% O by mass?
A) KSO2
B) KSO3
C) K2SO4
D) K2SO3
E) KSO4
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 178
Related Exams